
Chemistry, 12.03.2020 05:32 cxttiemsp021
A compound of molar mass 161 g/mol contains only carbon, hydrogen, bromine, and oxygen. Analysis reveals that the compound contains 12 times as much carbon as hydrogen by mass. Find the molecular formula. Express your answer as a chemical formula.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1

Chemistry, 23.06.2019 07:00
Introduction of drugs into the gastrointestinal tract is a form of administration. a. enteral b. topical c. parenteral d. inhalation
Answers: 1
You know the right answer?
A compound of molar mass 161 g/mol contains only carbon, hydrogen, bromine, and oxygen. Analysis rev...
Questions


Chemistry, 09.04.2020 21:57

History, 09.04.2020 21:57

Mathematics, 09.04.2020 21:57

History, 09.04.2020 21:57

History, 09.04.2020 21:57

Mathematics, 09.04.2020 21:57





Mathematics, 09.04.2020 21:57



History, 09.04.2020 21:57





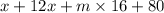 = 161
= 161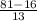
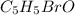 .
.


