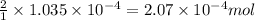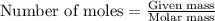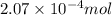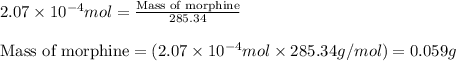Chemistry, 16.03.2020 18:45 zeldawhite76
Morphine has the formula C17H19NO3. It is a base and accepts one proton per molecule. It is isolated from opium. A 0.682g sample of opium is found to require 8.92 mL of a 0.0116 M solution of sulfuric acid for neutralization. Assuming that morphine is the only base present in opium, calculate the mass (in grams) of morphine in the sample of opium.
Reaction equation: 2 C17H19NO3 + H2SO4 --> Product

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

You know the right answer?
Morphine has the formula C17H19NO3. It is a base and accepts one proton per molecule. It is isolated...
Questions



English, 19.11.2019 16:31

Biology, 19.11.2019 16:31


Mathematics, 19.11.2019 16:31

History, 19.11.2019 16:31




Mathematics, 19.11.2019 16:31



History, 19.11.2019 16:31

Chemistry, 19.11.2019 16:31

Mathematics, 19.11.2019 16:31


Mathematics, 19.11.2019 16:31

Chemistry, 19.11.2019 16:31

Mathematics, 19.11.2019 16:31

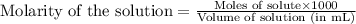
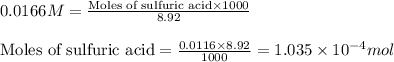
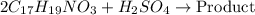
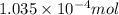 of sulfuric acid will react with =
of sulfuric acid will react with =