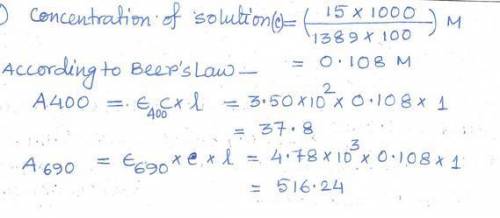In order to wash out the impurity, you looked up some sketchy method from 1952 which Hank Pym wrote during his Ph. D. under E. J. Corey. The method requires you add HCl to the mixture. After you rid your sample of any impurities, you take some pH paper and find out your solution has a pH of 3.0. Assuming the pKa of Pym Particles is 4.5 and that the molar absorptivity of the protonated Pym Particle is 4.82 * 102 L/mol*cm and 8.54 * 103 L/mol*cm for 400 nm and 690 nm respectively, calculate the expected A400 and A690 of the solution.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1


Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

You know the right answer?
In order to wash out the impurity, you looked up some sketchy method from 1952 which Hank Pym wrote...
Questions




Social Studies, 26.09.2019 00:10

Social Studies, 26.09.2019 00:10






English, 26.09.2019 00:10


English, 26.09.2019 00:10

English, 26.09.2019 00:10

English, 26.09.2019 00:10

History, 26.09.2019 00:10



Mathematics, 26.09.2019 00:10