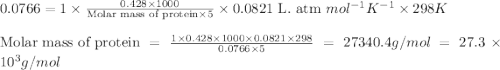Chemistry, 17.03.2020 05:41 meramera50
428. mg of an unknown protein are dissolved in enough solvent to make of solution. The osmotic pressure of this solution is measured to be at . Calculate the molar mass of the protein. Round your answer to significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 23.06.2019 09:30
The allotropes of carbon include a variety of structures that include three-dimensional tetrahedral lattices, planes of hexagonal rings, cylindrical tubes of hexagonal rings, and spheres of five- and six-membered rings. similar shapes of network covalent atomic solids are possible with carbon nitride, boron, and pure silicon (e.g., silicene is a graphene-like allotrope of pure silicon). in contrast, silicates exist as either highly ordered or amorphous (more random) three-dimensional lattices. what could explain why there are there no naturally occurring sheets, stacked sheets, cylindrical tubes, or spheres of network covalent atomic solids composed of silicon and oxygen (sio2)? would pure silicate structures make good lubricants or good electrical conductors?
Answers: 3
You know the right answer?
428. mg of an unknown protein are dissolved in enough solvent to make of solution. The osmotic press...
Questions





World Languages, 27.12.2021 14:00


Mathematics, 27.12.2021 14:00


Mathematics, 27.12.2021 14:10


Mathematics, 27.12.2021 14:10


History, 27.12.2021 14:10


Mathematics, 27.12.2021 14:20

Medicine, 27.12.2021 14:20

Computers and Technology, 27.12.2021 14:20

Mathematics, 27.12.2021 14:20

English, 27.12.2021 14:20

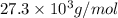
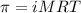
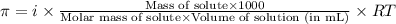
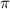 = osmotic pressure of the solution = 0.0766 atm
= osmotic pressure of the solution = 0.0766 atm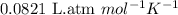
![25^oC=[273+25]=298K](/tpl/images/0550/3624/6a9f9.png)