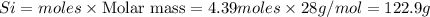The reaction of Cr2O3 with silicon metal at high temperatures will make chromium metal.
...

The reaction of Cr2O3 with silicon metal at high temperatures will make chromium metal.
2Cr2O3(s)+3Si(s) > 4Cr(l)+3SiO2(s)
The reaction is begun with 161.00 g of Si and 139.00 g of Cr2O3.
How many grams of the excess reactant is left after the reaction is complete?

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 01:30
Which statement accurately represents the arrangement of electrons in bohr’s atomic model?
Answers: 2


Chemistry, 23.06.2019 10:30
When a wire with a current is placed in a magnetic field, electrical energy is transformed into mechanical energy select the best answer from the choices provided t f
Answers: 2

Chemistry, 23.06.2019 21:10
Which of the following monomers is least likely to undergo cationic polymerization? a) propylene b) styrene c) methyl acrylate d) vinyl acetate e) isobutylene
Answers: 1
You know the right answer?
Questions

Health, 09.03.2021 19:30



Mathematics, 09.03.2021 19:30

History, 09.03.2021 19:30



Mathematics, 09.03.2021 19:30

Mathematics, 09.03.2021 19:30

Chemistry, 09.03.2021 19:30

Mathematics, 09.03.2021 19:30




History, 09.03.2021 19:30



Mathematics, 09.03.2021 19:30

Mathematics, 09.03.2021 19:30

Mathematics, 09.03.2021 19:30

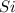 will be left from the given masses of both reactants.
will be left from the given masses of both reactants.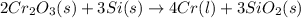
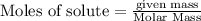
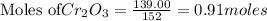
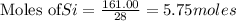
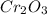 require 3 moles of
require 3 moles of 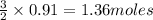 of
of