Chemistry, 19.03.2020 01:58 tristasbarker03
Calculate the volume of carbon dioxide at 20.0°C and 0.941 atm produced from the complete combustion of 4.00 kg of methane. Compare your result with the volume of CO2 produced from the complete combustion of 4.00 kg of propane (C3H8).

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1


Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
Calculate the volume of carbon dioxide at 20.0°C and 0.941 atm produced from the complete combustion...
Questions

Biology, 27.03.2020 06:31

Mathematics, 27.03.2020 06:31




Chemistry, 27.03.2020 06:31


History, 27.03.2020 06:31

Mathematics, 27.03.2020 06:31




Mathematics, 27.03.2020 06:31



Mathematics, 27.03.2020 06:31

History, 27.03.2020 06:31

Mathematics, 27.03.2020 06:31


Mathematics, 27.03.2020 06:32

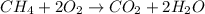
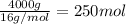
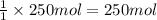 of carbon dioxide gas
of carbon dioxide gas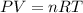 (Ideal gas equation)
(Ideal gas equation)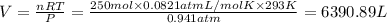
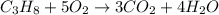
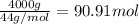
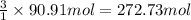 of carbon dioxide gas
of carbon dioxide gas