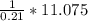A gas contains 75.0 wt% methane, 10.0% ethane, 5.0% ethylene, and the balance water. (a) Calculate the molar composition of this gas on both awet and a dry basis and the ratio (mol H2O/ mol dry gas). (b) If100kg/30%excessair,(kmol/ h)? How would the answer change if the combustion were only 75% complete?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1


Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
You know the right answer?
A gas contains 75.0 wt% methane, 10.0% ethane, 5.0% ethylene, and the balance water. (a) Calculate t...
Questions

English, 08.06.2021 21:50

Mathematics, 08.06.2021 21:50


English, 08.06.2021 21:50

Social Studies, 08.06.2021 21:50


English, 08.06.2021 21:50

Mathematics, 08.06.2021 21:50


Mathematics, 08.06.2021 21:50

Mathematics, 08.06.2021 21:50

Biology, 08.06.2021 21:50

Engineering, 08.06.2021 21:50

Chemistry, 08.06.2021 21:50

Mathematics, 08.06.2021 21:50

Mathematics, 08.06.2021 21:50

Mathematics, 08.06.2021 21:50

Mathematics, 08.06.2021 21:50

Chemistry, 08.06.2021 21:50

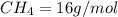
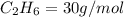
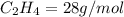
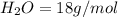
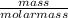
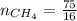
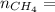 4.69 moles
4.69 moles 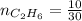
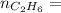 0.33 moles
0.33 moles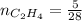
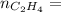 0.18 moles
0.18 moles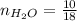
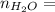 0.56 moles
0.56 moles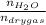
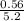




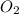 required = (2 × 4.69) + (3.5 × 0.33) + (3 × 0.18) k moles
required = (2 × 4.69) + (3.5 × 0.33) + (3 × 0.18) k moles