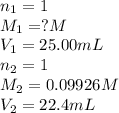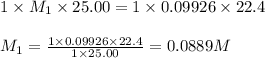Chemistry, 19.03.2020 18:34 wafflewarriormg
A 25.0 mL of a solution of acetic acid of unknown concentration is titrated with 0.09926 M NaOH and the equivalence point volume was determined by graphical means to be 22.4 mL. What is the concentration of the acetic acid?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
A 25.0 mL of a solution of acetic acid of unknown concentration is titrated with 0.09926 M NaOH and...
Questions






Mathematics, 25.02.2021 20:00

Computers and Technology, 25.02.2021 20:00

Mathematics, 25.02.2021 20:00




Chemistry, 25.02.2021 20:00


Mathematics, 25.02.2021 20:00

Mathematics, 25.02.2021 20:00



Mathematics, 25.02.2021 20:00


Mathematics, 25.02.2021 20:00

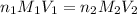
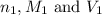 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 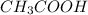
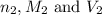 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.