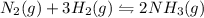Chemistry, 19.03.2020 20:43 lovelylife7553
The reaction for the synthesis of ammonia N2(g) + 3 H2(g) → 2 NH3(g) is exothermic. Increasing the temperature applied to the system I) increases the amount of NH3. II) decreases the amount of NH3. III) changes the value of Keq. IV) does not change the value of Keq.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:00
How has the scientific community addressed the safety of chemicals? a. chemicals are repeatedly tested, even those that have existed for a long time. b. existing chemicals are tested if they have never been tested before. c. chemicals are tested if they are suspected to have caused a problem. d. only new chemicals are tested.
Answers: 2

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
The reaction for the synthesis of ammonia N2(g) + 3 H2(g) → 2 NH3(g) is exothermic. Increasing the t...
Questions


Mathematics, 22.08.2020 14:01

Chemistry, 22.08.2020 14:01


Mathematics, 22.08.2020 14:01




Computers and Technology, 22.08.2020 14:01


Mathematics, 22.08.2020 14:01

Mathematics, 22.08.2020 14:01



Mathematics, 22.08.2020 14:01

Computers and Technology, 22.08.2020 14:01