
Chemistry, 20.03.2020 01:32 samymaria1344
At a certain temperature, 0.780 mol SO 3 0.780 mol SO3 is placed in a 4.00 L 4.00 L container. 2 SO 3 ( g ) − ⇀ ↽ − 2 SO 2 ( g ) + O 2 ( g ) 2SO3(g)↽−−⇀2SO2(g)+O2(g) At equilibrium, 0.100 mol O 2 0.100 mol O2 is present. Calculate K c .

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Select the correct answer. what is the nature of the se-cl bond in a molecule of selenium chloride (secl2) if the electronegativity value of selenium is 2.55 and that of chlorine is 3.16?
Answers: 3

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
You know the right answer?
At a certain temperature, 0.780 mol SO 3 0.780 mol SO3 is placed in a 4.00 L 4.00 L container. 2 SO...
Questions


Mathematics, 19.02.2020 04:57

Mathematics, 19.02.2020 04:57





History, 19.02.2020 04:57

Computers and Technology, 19.02.2020 04:57





Mathematics, 19.02.2020 04:57




Social Studies, 19.02.2020 04:57

Mathematics, 19.02.2020 04:57

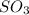 = 0.780 mole
= 0.780 mole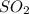 =
= 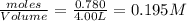
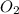 =
= 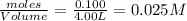
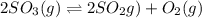
![K_c=\frac{[SO_2]^2\times [O_2]}{[SO_3]^2}](/tpl/images/0555/1079/d826c.png)
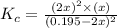
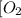 = x = 0.025 M
= x = 0.025 M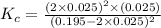
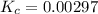
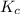 is 0.00297
is 0.00297 

