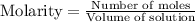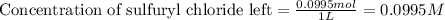Chemistry, 20.03.2020 09:55 solobiancaa
The decomposition of sulfuryl chloride into sulfur dioxide and chlorine SO2Cl2(g) → SO2(g) + Cl2(g) follows first-order kinetics. At 320◦C the rate constant is 2.2 × 10−5 sec−1 . If one started with a sample containing 0.16 moles of sulfuryl chloride per liter at 320◦C, what concentration would be left after 6.00 hours?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
The decomposition of sulfuryl chloride into sulfur dioxide and chlorine SO2Cl2(g) → SO2(g) + Cl2(g)...
Questions


History, 24.05.2020 22:57



Mathematics, 24.05.2020 22:57



World Languages, 24.05.2020 22:57


History, 24.05.2020 22:57


Mathematics, 24.05.2020 22:57

Mathematics, 24.05.2020 22:57

English, 24.05.2020 22:57


Mathematics, 24.05.2020 22:57


Mathematics, 24.05.2020 22:57

Mathematics, 24.05.2020 22:57

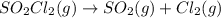
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0555/8016/f1041.png)
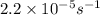
![[A_o]](/tpl/images/0555/8016/dc622.png) = initial amount of the sample = 0.16 moles
= initial amount of the sample = 0.16 moles![2.2\times 10^{-5}=\frac{2.303}{21600}\log\frac{0.16}{[A]}](/tpl/images/0555/8016/ba4a8.png)
![[A]=0.0995moles](/tpl/images/0555/8016/152d1.png)