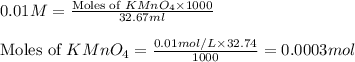Chemistry, 20.03.2020 10:46 jdisalle476
A sample of an Iron Oxalato complex salt weighting 0.13 grams requires 32.74 mL of 0.01 M KMnO4 to turn the solution a very light pink color at the quivalence point. Calculate the number of moles of KMnO4 added.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

You know the right answer?
A sample of an Iron Oxalato complex salt weighting 0.13 grams requires 32.74 mL of 0.01 M KMnO4 to t...
Questions



Chemistry, 20.09.2019 06:20


English, 20.09.2019 06:20




Mathematics, 20.09.2019 06:20

Mathematics, 20.09.2019 06:20


Social Studies, 20.09.2019 06:20

Mathematics, 20.09.2019 06:20


Health, 20.09.2019 06:20

History, 20.09.2019 06:20

History, 20.09.2019 06:20

History, 20.09.2019 06:20

History, 20.09.2019 06:20

Chemistry, 20.09.2019 06:20

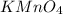 added are 0.0003
added are 0.0003 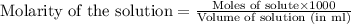 .....(1)
.....(1)