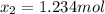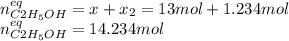Chemistry, 23.03.2020 23:20 little68941
While ethanol (CH3CH2OH) is produced naturally by fermentation, e. g. in beer- and wine-making, industrially it is synthesized by reacting ethylene (CH2CH2) with water vapor at elevated temperatures A chemical engineer studying this reaction fills a 75.0 L tank at 18. °C with 29. mol of ethylene gas and 16. mol of water vapor. He then raises the temperature considerably, and when the mixture has come to equilibrium determines that it contains 16. mol of ethylene gas and 3.0 mol of water vapor. 囲 The engineer then adds another 15. mol of ethylene, and allows the mixture to come to equilibrium again. Calculate the moles of ethanol after equilibrium is reached the second time. Round your answer to 2 significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1


Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2
You know the right answer?
While ethanol (CH3CH2OH) is produced naturally by fermentation, e. g. in beer- and wine-making, indu...
Questions






Chemistry, 12.02.2020 19:24


Mathematics, 12.02.2020 19:24



English, 12.02.2020 19:24

Mathematics, 12.02.2020 19:24

History, 12.02.2020 19:24

History, 12.02.2020 19:25


Social Studies, 12.02.2020 19:25


Social Studies, 12.02.2020 19:25

Biology, 12.02.2020 19:25

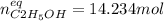
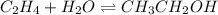
![Kc=\frac{[CH_3CH_2OH]_{eq}}{[H_2O]_{eq}[CH_2CH_2]_{eq}}](/tpl/images/0559/8704/4cbd4.png)
 result:
result:![[CH_2CH_2]_{eq}=29mol-x=16mol\\x=29-16=13mol](/tpl/images/0559/8704/ac34b.png)
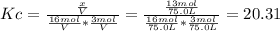
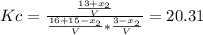
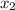 finally result (solving by solver or quadratic equation):
finally result (solving by solver or quadratic equation):