
Chemistry, 26.03.2020 05:28 Guidomigoo3x
A buffer consists of 0.120 m hno2 and 0.150 m nano2 at 25°c.
a. what is the ph of the buffer?
b. what is the ph after the addition of 1.00 ml of 11.6 m hcl to 1.00 l of the buffer solution?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
A buffer consists of 0.120 m hno2 and 0.150 m nano2 at 25°c.
a. what is the ph of the bu...
a. what is the ph of the bu...
Questions









Social Studies, 05.09.2019 17:20




English, 05.09.2019 17:20

Mathematics, 05.09.2019 17:20

Business, 05.09.2019 17:20



Mathematics, 05.09.2019 17:20

Mathematics, 05.09.2019 17:20

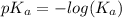
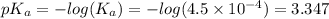
![pH = pK_{a} + log\frac{[conjugate base]}{[acid]}](/tpl/images/0564/8674/1bac7.png)
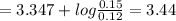
 will react with
will react with 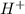 to form
to form
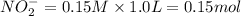
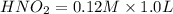
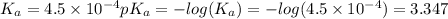
![pH = pK_{a} + log \frac{[conjugate base]}{[acid]}](/tpl/images/0564/8674/401ef.png)



