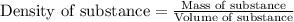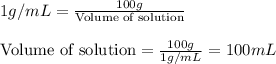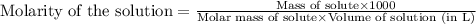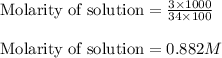Hydrogen peroxide, a disinfectant, contains 3.0% (w/w) hydrogen peroxide in water. This means there are 3.0 grams of hydrogen peroxide in every 100. grams of solution. Assuming this solution has a density of 1.00 g/mL, what is the molar concentration of this solution

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3

Chemistry, 23.06.2019 10:10
Solid tin exists in two forms: white and gray. for the transformation sn(s, white) → sn(s, gray) the enthalpy change is -2.1 kj/mol and the entropy change is -7.4 j/(mol*k). a. calculate the gibbs free energy change for the conversion of 1.00 mol white tin to gray tin at -30℃. b. will white tin convert spontaneously to gray tin at -30℃? c. at what temperature are white and gray tin thermodynamically equivalent at a pressure of 1 atm?
Answers: 3

Chemistry, 23.06.2019 20:00
(05.04 mc) the table below shows the set of traits in four different beetle populations. traits in population population traits 1 brown-colored beetles, pesticide-resistant gene present 2 green-colored beetles, pesticide-resistant gene absent 3 green and brown–colored beetles, pesticide-resistant gene absent 4 green and brown−colored beetles, pesticide-resistant gene present which population of beetles is most likely to become extinct when the existing conditions change? (4 points) select one: a. 1 b. 2 c. 3 d. 4
Answers: 1

Chemistry, 24.06.2019 01:30
Atest within an experiment that is conducted under standard conditions is called a. control b. benchmark c. standard reaction d. all of the above e. none of the above
Answers: 1
You know the right answer?
Hydrogen peroxide, a disinfectant, contains 3.0% (w/w) hydrogen peroxide in water. This means there...
Questions

History, 02.07.2019 15:00

Mathematics, 02.07.2019 15:00


Mathematics, 02.07.2019 15:00

Chemistry, 02.07.2019 15:00

Chemistry, 02.07.2019 15:00



English, 02.07.2019 15:00

Social Studies, 02.07.2019 15:00

Chemistry, 02.07.2019 15:00


Social Studies, 02.07.2019 15:00





Mathematics, 02.07.2019 15:00

History, 02.07.2019 15:00