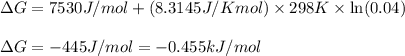Chemistry, 30.03.2020 21:38 zhuotingwu147
For the aqueous reaction dihydroxyacetone phosphate is the reactant and glyceraldehyde 3 phosphate is the product. dihydroxyacetone phosphate − ⇀ ↽ − glyceraldehyde − 3 − phosphate the standard change in Gibbs free energy is Δ G ° ' = 7.53 kJ/mol . Calculate Δ G for this reaction at 298 K when [dihydroxyacetone phosphate] = 0.100 M and [glyceraldehyde-3-phosphate] = 0.00400 M . Δ G = kJ / mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
You know the right answer?
For the aqueous reaction dihydroxyacetone phosphate is the reactant and glyceraldehyde 3 phosphate i...
Questions


English, 24.01.2020 10:31


Mathematics, 24.01.2020 10:31


Mathematics, 24.01.2020 10:31

Mathematics, 24.01.2020 10:31

Mathematics, 24.01.2020 10:31

History, 24.01.2020 10:31


Mathematics, 24.01.2020 10:31





Mathematics, 24.01.2020 10:31


Social Studies, 24.01.2020 10:31


Advanced Placement (AP), 24.01.2020 10:31

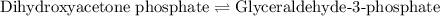
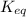 of above equation is:
of above equation is:![K_{eq}=\frac{\text{[Glyceraldehyde-3-phosphate]}}{\text{[Dihydroxyacetone phosphate]}}](/tpl/images/0571/3632/f265a.png)
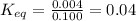

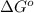 = Standard Gibbs free energy = 7.53 kJ/mol = 7530 J/mol
= Standard Gibbs free energy = 7.53 kJ/mol = 7530 J/mol