
Chemistry, 30.03.2020 21:22 usagimiller
Coal gasification can be represented by the equation: 2 C(s) + 2 H2O(g) → CH4(g) + CO2(g) ΔH = ? Use the following information to find ΔH for the reaction above. CO(g) + H2(g) → C(s) + H2O(g) ΔH = -131 kJ CO(g) + H2O(g) → CO2(g) + H2(g) ΔH = -41 kJ CO(g) + 3 H2(g) → CH4(g) + H2O(g) ΔH = -206 kJ

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
Coal gasification can be represented by the equation: 2 C(s) + 2 H2O(g) → CH4(g) + CO2(g) ΔH = ? Use...
Questions

Mathematics, 25.07.2019 11:10

Physics, 25.07.2019 11:10

Mathematics, 25.07.2019 11:10

Mathematics, 25.07.2019 11:10


Mathematics, 25.07.2019 11:10

Geography, 25.07.2019 11:10

Mathematics, 25.07.2019 11:10


Social Studies, 25.07.2019 11:10

Biology, 25.07.2019 11:10

Biology, 25.07.2019 11:10

Biology, 25.07.2019 11:10

Biology, 25.07.2019 11:10


Mathematics, 25.07.2019 11:10



English, 25.07.2019 11:10

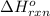 for the reaction is 15 kJ.
for the reaction is 15 kJ.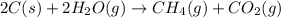
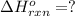
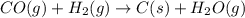
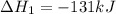 ( × 2)
( × 2)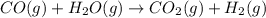
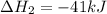
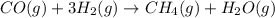
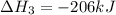
![\Delta H^o_{rxn}=[2\times (-\Delta H_1)]+[1\times \Delta H_2]+[1\times \Delta H_3]](/tpl/images/0571/2803/fd23d.png)
![\Delta H^o_{rxn}=[(2\times -(-131))+(1\times (-41))+(1\times (-206))]=15kJ](/tpl/images/0571/2803/ea721.png)


