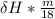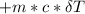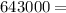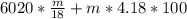Chemistry, 30.03.2020 22:31 jaccamoboy
A backpacker collects snow at 0°C, and places it in a cooking pot on a camp stove. It takes 643 kJ of heat energy to melt the snow and bring the water to boiling. Assuming no heat loss, and neglecting the specific heat capacity of the pot, calculate the mass of snow that the backpacker collected. (Data: specific heat capacity of liquid water, c = 4.18 J/g⋅K; and: H2O(s) → H2O(l) ΔH = ΔHfusion = 6.02 kJ/mol)

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1
You know the right answer?
A backpacker collects snow at 0°C, and places it in a cooking pot on a camp stove. It takes 643 kJ o...
Questions



History, 21.04.2021 19:50

Biology, 21.04.2021 19:50

Mathematics, 21.04.2021 19:50

Mathematics, 21.04.2021 19:50




Mathematics, 21.04.2021 19:50

Chemistry, 21.04.2021 19:50


History, 21.04.2021 19:50

Mathematics, 21.04.2021 19:50


Chemistry, 21.04.2021 19:50



Mathematics, 21.04.2021 19:50

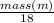
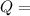
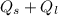
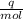
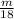 for the number of moles; so:
for the number of moles; so: