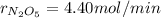Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

You know the right answer?
The decomposition of dinitrogen pentoxide is described by the chemical equation 2 N2O5(g) → 4 NO2(g)...
Questions



Computers and Technology, 27.04.2021 23:20



Biology, 27.04.2021 23:20

Arts, 27.04.2021 23:20





Mathematics, 27.04.2021 23:30


Computers and Technology, 27.04.2021 23:30

Engineering, 27.04.2021 23:30

Mathematics, 27.04.2021 23:30

Geography, 27.04.2021 23:30

World Languages, 27.04.2021 23:30

Mathematics, 27.04.2021 23:30

History, 27.04.2021 23:30