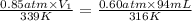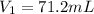Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
You know the right answer?
An ideal gas originally at 0.85 atm and 66°C was allowed to expand until its final volume, pressure,...
Questions


English, 18.10.2021 07:20

Spanish, 18.10.2021 07:20

Mathematics, 18.10.2021 07:20


Spanish, 18.10.2021 07:20

History, 18.10.2021 07:20





Spanish, 18.10.2021 07:20

Mathematics, 18.10.2021 07:20





Social Studies, 18.10.2021 07:20


Spanish, 18.10.2021 07:20

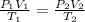
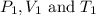 are the initial pressure, volume and temperature of the gas
are the initial pressure, volume and temperature of the gas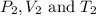 are the final pressure, volume and temperature of the gas
are the final pressure, volume and temperature of the gas![P_1=0.85atm\\V_1=?\\T_1=66^oC=[66+273]K=339K\\P_2=0.60atm\\V_2=94mL\\T_2=43^oC=[43+273]K=316K](/tpl/images/0571/6562/d1207.png)