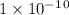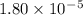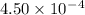Chemistry, 31.03.2020 04:19 jessicaflower277
Given the following acids and their Ka values: Phenol, C6H5OH Ka = 1.00 × 10-10 Acetic acid, CH3CO2H Ka = 1.80 × 10-5 Nitrous acid, HNO2 Ka = 4.50 × 10-4 What is the order of increasing base strength for C6H5O−, CH3COO−, and NO2−? Group of answer choices

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2

Chemistry, 23.06.2019 06:30
(04.01 lc) which of the following is true about science? (5 points) select one: a. it is not influenced by social conditions. b. it is not determined by external local factors. c. political conditions are unable to influence it. d. economic concerns may prevent it from solving problems.
Answers: 1
You know the right answer?
Given the following acids and their Ka values: Phenol, C6H5OH Ka = 1.00 × 10-10 Acetic acid, CH3CO2H...
Questions

Chemistry, 02.04.2020 23:30

Social Studies, 02.04.2020 23:30



Mathematics, 02.04.2020 23:30





Mathematics, 02.04.2020 23:30


Chemistry, 02.04.2020 23:30

Mathematics, 02.04.2020 23:30

Biology, 02.04.2020 23:30

Mathematics, 02.04.2020 23:30


Mathematics, 02.04.2020 23:30



Mathematics, 02.04.2020 23:30

 <
< 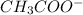 <
< 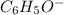
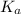 value is a measure of acidic strength. The larger the
value is a measure of acidic strength. The larger the