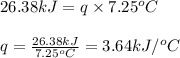Chemistry, 01.04.2020 01:20 hosteenimport21
What is the heat capacity of the calorimeter, Calorimeter, (in kJ/oC) of a bomb calorimeter if burning 1.0 g of benzoic acid in it causes the temperature to rise by 7.25 oC? Given ΔHcomb = LaTeX: -−26.38 kJ/g.
Answer Choices:
3221.53
3.64
191.26
25.34
-3.64

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1
You know the right answer?
What is the heat capacity of the calorimeter, Calorimeter, (in kJ/oC) of a bomb calorimeter if burni...
Questions




Mathematics, 25.04.2020 04:42


Computers and Technology, 25.04.2020 04:42




Physics, 25.04.2020 04:43

Arts, 25.04.2020 04:43






History, 25.04.2020 04:43


History, 25.04.2020 04:43

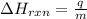
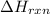 = enthalpy change of the reaction = -26.38 kJ/g
= enthalpy change of the reaction = -26.38 kJ/g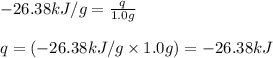
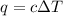
 = change in temperature = 7.25°C
= change in temperature = 7.25°C