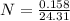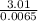Chemistry, 04.04.2020 12:39 Savageman4654
In an experiment to determine the enthalpy change for this reaction, you combine 0.158 g of Mg metal with enough HCl to make 100.0 mL of solution in a coffee-cup calorimeter. The HCl is sufficiently concentrated so that the Mg completely reacts. The temperature of the solution rises from 25.6 °C to 32.8 °C as a result of the reaction. Find ΔHrxn for the reaction as written. Use 1.00 g/mL as the dens

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 23.06.2019 18:40
Sound does not need a medium to travel is reflected when it bounces off a shiny surface is created by electric and magnetic fields moves in longitudinal waves
Answers: 1

Chemistry, 23.06.2019 19:30
(04.03 lc) which of the following is released during cellular respiration? select one: a. carbon dioxide b. glucose c. mitochondria d. oxygen
Answers: 2

Chemistry, 23.06.2019 23:10
An object was measured by a worker as 14.6cm long, however, the manufacturer specifications list the length of the object at 14.4cm. what is the percent error in the worker's measurement?
Answers: 1
You know the right answer?
In an experiment to determine the enthalpy change for this reaction, you combine 0.158 g of Mg metal...
Questions

History, 10.12.2020 22:40



Mathematics, 10.12.2020 22:40

Mathematics, 10.12.2020 22:40


Biology, 10.12.2020 22:40

English, 10.12.2020 22:40

Mathematics, 10.12.2020 22:40

Business, 10.12.2020 22:40



Mathematics, 10.12.2020 22:40

Mathematics, 10.12.2020 22:40

Mathematics, 10.12.2020 22:40

Mathematics, 10.12.2020 22:40



Mathematics, 10.12.2020 22:40

Spanish, 10.12.2020 22:40

 for the reaction = 463
for the reaction = 463 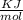
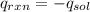
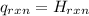
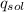 ------ (1)
------ (1)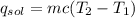
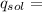 (1)(100) × 4.18 × (32.8 - 25.6)
(1)(100) × 4.18 × (32.8 - 25.6)