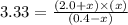Chemistry, 07.04.2020 02:47 lathwkuster
At certain temperature, kc for the reaction, PCl5 > PCl3 + Cl2, is equal to 3.33. After .20 mole of PCL5 and 1.0 mole of PCL3 are introduced iinto a 2.00 L evacuated chamber, calculate the equilibrium concentration of PCl5.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
At certain temperature, kc for the reaction, PCl5 > PCl3 + Cl2, is equal to 3.33. After .20 mole...
Questions


Mathematics, 27.02.2021 14:00


Mathematics, 27.02.2021 14:00

English, 27.02.2021 14:00

Social Studies, 27.02.2021 14:00

Engineering, 27.02.2021 14:00

Mathematics, 27.02.2021 14:00

History, 27.02.2021 14:00

Mathematics, 27.02.2021 14:00


Spanish, 27.02.2021 14:00

Mathematics, 27.02.2021 14:00

Spanish, 27.02.2021 14:00


Mathematics, 27.02.2021 14:00




Chemistry, 27.02.2021 14:00

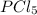 is, 0.16 M
is, 0.16 M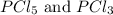
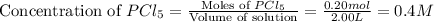
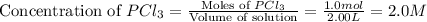
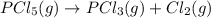
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0585/7341/73fe0.png)