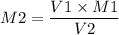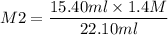Chemistry, 07.04.2020 21:31 noathequeen
If I have 15.40mL of 1.4M HBr(aq) and add 22.10mL KOH what is the molarity of
KOH being used?

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3

Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3

Chemistry, 23.06.2019 12:50
Acertain reaction has a activation energy of 54.0 kj/mol. as the temperature is increased from 22c to a higher temperature, the rate constant increases by a factor of 7.00. calculate the higher temperature. c (report only numerical answer)
Answers: 3
You know the right answer?
If I have 15.40mL of 1.4M HBr(aq) and add 22.10mL KOH what is the molarity of
KOH being used?...
KOH being used?...
Questions


Chemistry, 11.10.2019 08:31


History, 11.10.2019 08:31

Physics, 11.10.2019 08:31







Mathematics, 11.10.2019 08:31



Mathematics, 11.10.2019 08:50

Mathematics, 11.10.2019 08:50

Biology, 11.10.2019 08:50

Biology, 11.10.2019 08:50

Mathematics, 11.10.2019 08:50