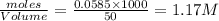Chemistry, 08.04.2020 01:53 Abdirisack3250
Calculate the mass of AgCl formed, and the concentration of silver ion remaining in solution, when 10.0g of solid AgNO3 is added to 50.mL of 1.0x10-2 Assume there is no volume change upon addition of the solid.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
Calculate the mass of AgCl formed, and the concentration of silver ion remaining in solution, when 1...
Questions


Mathematics, 28.10.2019 20:31

Mathematics, 28.10.2019 20:31



Mathematics, 28.10.2019 20:31







Social Studies, 28.10.2019 20:31

Business, 28.10.2019 20:31




Mathematics, 28.10.2019 20:31


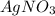 is added to 50.mL of
is added to 50.mL of 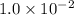 NaCl. Assume there is no volume change upon addition of the solid.
NaCl. Assume there is no volume change upon addition of the solid.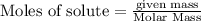
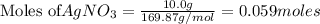
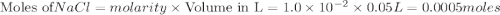
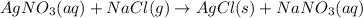
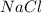 require = 1 mole of
require = 1 mole of 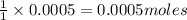 of
of 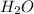
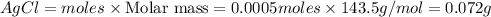
![[Ag]^+](/tpl/images/0588/5138/ed5fe.png) left in solution =
left in solution =