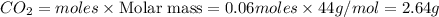G Gaseous methane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 0.96 g of methane is mixed with 6.37 g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
What is a scientific theory? a. a scientist's guess about how something works b. the results of an experiment obtained using the scientific method c. a proven fact that will never change d. an idea that is backed by data from many sources
Answers: 2

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1
You know the right answer?
G Gaseous methane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water...
Questions

History, 28.01.2020 07:31





English, 28.01.2020 08:31


Geography, 28.01.2020 08:31

Biology, 28.01.2020 08:31


History, 28.01.2020 08:31

Mathematics, 28.01.2020 08:31

Mathematics, 28.01.2020 08:31

Biology, 28.01.2020 08:31

Mathematics, 28.01.2020 08:31

Mathematics, 28.01.2020 08:31

Spanish, 28.01.2020 08:31

Physics, 28.01.2020 08:31

Mathematics, 28.01.2020 08:31

History, 28.01.2020 08:31

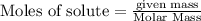
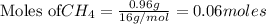
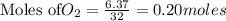
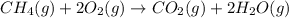
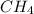 require = 2 moles of
require = 2 moles of 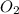
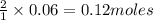 of
of 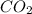
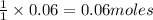 of
of