
Chemistry, 08.04.2020 17:23 narwalmaster2001
Hydrogen (H2) combines with chlorine (Cl2) to form hydrochloric acid (HCl): H2 + Cl2 → 2HCl. Using the chart below, what is the estimated enthalpy change for this reaction? (Assume no changes in pressure or volume.)
A. 494 kJ/mol
B. 247 kJ/mol
C. –58 kJ/mol
D. –184 kJ/mol
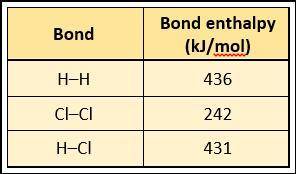

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
Hydrogen (H2) combines with chlorine (Cl2) to form hydrochloric acid (HCl): H2 + Cl2 → 2HCl. Using t...
Questions






Mathematics, 13.07.2019 11:30

Mathematics, 13.07.2019 11:30

Mathematics, 13.07.2019 11:30

Arts, 13.07.2019 11:30





Mathematics, 13.07.2019 11:30



Mathematics, 13.07.2019 11:30


Biology, 13.07.2019 11:30

Chemistry, 13.07.2019 11:30


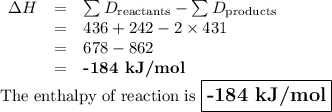 .
.


