
Chemistry, 08.04.2020 18:22 fatlip2429
Calculate the change in enthalpy (LaTeX: \DeltaΔH) of a reaction with the following information:
Energy of Reactants = 129 kJ
Energy of the Products = 110 kJ
Energy of the Transition State = 198 kJ

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 23.06.2019 07:00
Ajar contains a certain substance. which observation would show that the substance must be either a solid or a liquid?
Answers: 1

Chemistry, 23.06.2019 07:10
Which one of the following is an oxidation-reduction reaction? naoh + hno3 --> h2o + kno3 naoh + hno3 --> h2o + kno3 so3 + h2o --> h2so4 cacl2 + na2co3 --> caco3 + 2 nacl ch4 + 2 o2 --> co2 + 2 h2o al2(so4)3 + 6 koh --> 2 al(oh)3 + 3 k2so4
Answers: 3

Chemistry, 23.06.2019 19:30
How has the scientific model of the atom changed over the centuries, and what new evidence led to the various changes in the model?
Answers: 1
You know the right answer?
Calculate the change in enthalpy (LaTeX: \DeltaΔH) of a reaction with the following information:
Questions

Mathematics, 22.05.2020 09:59




English, 22.05.2020 09:59

Mathematics, 22.05.2020 09:59

English, 22.05.2020 09:59




Social Studies, 22.05.2020 09:59




Mathematics, 22.05.2020 10:00

English, 22.05.2020 10:00



History, 22.05.2020 10:00

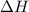 is mathematically expressed as the difference between the the total potential energy of products
is mathematically expressed as the difference between the the total potential energy of products 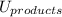 and the potential energy of the reactants
and the potential energy of the reactants 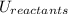 :
: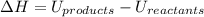
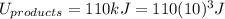
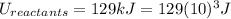
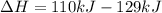
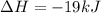 The negative sign in this result means we have a negative enthalpy, hence an exothermic reaction (where heat is released).
The negative sign in this result means we have a negative enthalpy, hence an exothermic reaction (where heat is released). 

