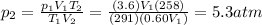Chemistry, 08.04.2020 19:24 pedroramirezr2
For many purposes we can treat nitrogen N2 as an ideal gas at temperatures above its boiling point of −196.°C. Suppose the temperature of a sample of nitrogen gas is lowered from 18.0°C to −15.0°C, and at the same time the pressure is changed. If the initial pressure was 3.6atm and the volume decreased by 40.0%, what is the final pressure? Round your answer to the correct number of significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:40
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b.zinc7.14,c.copper 8.92,d.lead 11.34
Answers: 2

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
For many purposes we can treat nitrogen N2 as an ideal gas at temperatures above its boiling point o...
Questions


Social Studies, 14.06.2021 21:00

Mathematics, 14.06.2021 21:00

English, 14.06.2021 21:00

Mathematics, 14.06.2021 21:00

Mathematics, 14.06.2021 21:00

Mathematics, 14.06.2021 21:00











Mathematics, 14.06.2021 21:00

Mathematics, 14.06.2021 21:00

Spanish, 14.06.2021 21:00

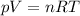

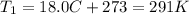 is the initial temperature of the gas
is the initial temperature of the gas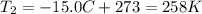 is the final temperature
is the final temperature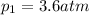 is the initial pressure
is the initial pressure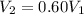 , as the volume is decreased by 40.0%
, as the volume is decreased by 40.0%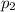 , we find the final pressure of the gas:
, we find the final pressure of the gas: