
Chemistry, 09.04.2020 03:31 jeisleen6808
2NH3(g) CO2(g) In an experiment carried out at this temperature, a certain amount of NH4OCONH2 is placed in an evacuated rigid container and allowed to come to equilibrium. Calculate the total pressure in the container at equilibrium.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:30
An occluded front moves over the farmland that has been experiencing drought conditions. what change in weather will this front likely bring? a. gray skies, but no rain b. an extended period of rain c. more dry air and sunny skies d. violent, short-lived thunderstorms
Answers: 3

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1


Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1
You know the right answer?
2NH3(g) CO2(g) In an experiment carried out at this temperature, a certain amount of NH4OCONH2 is pl...
Questions


Mathematics, 02.10.2020 09:01


English, 02.10.2020 09:01


History, 02.10.2020 09:01

Chemistry, 02.10.2020 09:01

Biology, 02.10.2020 09:01

Mathematics, 02.10.2020 09:01

Social Studies, 02.10.2020 09:01

Social Studies, 02.10.2020 09:01

Mathematics, 02.10.2020 09:01


Mathematics, 02.10.2020 09:01




Mathematics, 02.10.2020 09:01

Mathematics, 02.10.2020 09:01

Spanish, 02.10.2020 09:01

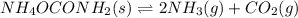
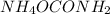 be 'x'
be 'x'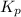 for above equation follows:
for above equation follows: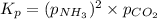
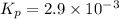
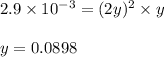
![p_{NH_3}+p_{CO_2}=[0.1796+0.0898]=0.2694atm](/tpl/images/0591/2646/0c04d.png)


