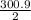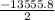Chemistry, 11.04.2020 01:50 smileyjesse6073
Calculate the enthalpy of combustion of 1 mol decane, C10H22, (l), to form CO2 and H2O. ∆Hf0 for decane is —300.9 kJ/mol using the balanced chemical equation and Appendix Table B–14.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1



Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
You know the right answer?
Calculate the enthalpy of combustion of 1 mol decane, C10H22, (l), to form CO2 and H2O. ∆Hf0 for dec...
Questions






Mathematics, 15.11.2019 23:31














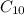
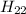 + 31
+ 31 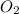 ⇒ 20 C
⇒ 20 C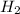 O
O