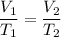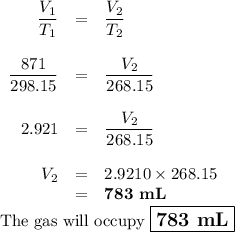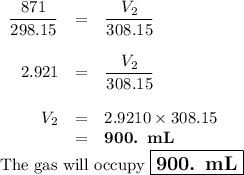Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 23.06.2019 09:30
What lessons does the history and study of the periodic table offer to other fields of science, and the pursuit science more generally
Answers: 3

Chemistry, 23.06.2019 10:20
An engineer wishes to design a container that will hold 12.0 mol of ethane at a pressure no greater than 5.00x10*2 kpa and a temperature of 52.0 degrees celsius. what is the minimum volume the container can have?
Answers: 1
You know the right answer?
A sample of O2 gas occupies a volume of 871 mL at 25 °C. If pressure remains constant, what would be...
Questions

Mathematics, 21.02.2022 03:30


Mathematics, 21.02.2022 03:30



Computers and Technology, 21.02.2022 03:30

English, 21.02.2022 03:30




Mathematics, 21.02.2022 03:30

Biology, 21.02.2022 03:30


Mathematics, 21.02.2022 03:30



Mathematics, 21.02.2022 03:40