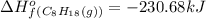Chemistry, 14.04.2020 19:48 rileyallen4186pd5tgy
For a particular isomer of C 8 H 18 , the combustion reaction produces 5093.7 kJ of heat per mole of C 8 H 18 ( g ) consumed, under standard conditions. C 8 H 18 ( g ) + 25 2 O 2 ( g ) ⟶ 8 CO 2 ( g ) + 9 H 2 O ( g ) Δ H ∘ rxn = − 5093.7 kJ / mol What is the standard enthalpy of formation of this isomer of C 8 H 18 ( g ) ?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
For a particular isomer of C 8 H 18 , the combustion reaction produces 5093.7 kJ of heat per mole of...
Questions


History, 21.03.2020 02:57

Geography, 21.03.2020 02:57


Mathematics, 21.03.2020 02:57

Mathematics, 21.03.2020 02:57

Biology, 21.03.2020 02:57

Health, 21.03.2020 02:57

English, 21.03.2020 02:57



Mathematics, 21.03.2020 02:57

Mathematics, 21.03.2020 02:57

Mathematics, 21.03.2020 02:57

Computers and Technology, 21.03.2020 02:57

History, 21.03.2020 02:57



English, 21.03.2020 02:57

Mathematics, 21.03.2020 02:57

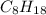 is -230.68 kJ/mol
is -230.68 kJ/mol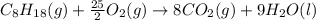
![\Delta H^o_{rxn}=[(8\times \Delta H^o_f_{(CO_2(g))})+(9\times \Delta H^o_f_{(H_2O(g))})]-[(1\times \Delta H^o_f_{(C_8H_{18}(g))})+(\frac{25}{2}\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0598/9874/d63c1.png)
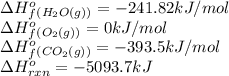
![-5093.7=[(8\times (-393.5))+(9\times (-241.82))]-[(1\times \Delta H^o_f_{(C_8H_{18}(g))})+(\frac{25}{2}\times (0))](/tpl/images/0598/9874/d83e1.png)