
A 9.75 L 9.75 L container holds a mixture of two gases at 41 ° C. 41 °C. The partial pressures of gas A and gas B, respectively, are 0.419 atm 0.419 atm and 0.589 atm. 0.589 atm. If 0.240 mol 0.240 mol of a third gas is added with no change in volume or temperature, what will the total pressure become?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 23.06.2019 02:50
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3
You know the right answer?
A 9.75 L 9.75 L container holds a mixture of two gases at 41 ° C. 41 °C. The partial pressures of ga...
Questions

Social Studies, 15.11.2019 19:31






Mathematics, 15.11.2019 19:31


Mathematics, 15.11.2019 19:31


Mathematics, 15.11.2019 19:31


Mathematics, 15.11.2019 19:31





Mathematics, 15.11.2019 19:31


Mathematics, 15.11.2019 19:31

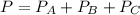 ----- (1)
----- (1)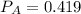 atm
atm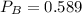 atm
atm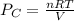
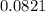
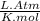
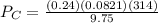
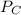 = 0.634 atm
= 0.634 atm

