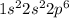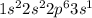Chemistry, 15.04.2020 00:24 twinkle713derp
7.47 Two atoms have the electron configurations 1s22s22p6 and 1s22s22p63s1. The first ionization energy of one is 2080 kJ/mol and that of the other is 496 kJ/mol. Match each ionization energy with one of the given electron configurations.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 06:30
Which of these natural resources is non-renewable a.corn b.wind c.geothermal d.natural gas
Answers: 2
You know the right answer?
7.47 Two atoms have the electron configurations 1s22s22p6 and 1s22s22p63s1. The first ionization ene...
Questions

Mathematics, 02.09.2019 10:10


Chemistry, 02.09.2019 10:10



Mathematics, 02.09.2019 10:10

History, 02.09.2019 10:10


Chemistry, 02.09.2019 10:10

Biology, 02.09.2019 10:10

Mathematics, 02.09.2019 10:10

History, 02.09.2019 10:10

Mathematics, 02.09.2019 10:10




Business, 02.09.2019 10:10



Social Studies, 02.09.2019 10:10