
Chemistry, 15.04.2020 00:52 calebcoolbeans6691
A chemist titrates 160.0 mL of a 0.3065 M cyanic acid (HCNO) solution with 0.4994 M NaOH solution at 25 °C. Calculate the pH at equivalence. The pK of cyanic acid is 3.46 Round your answer to 2 decimal places.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1


Chemistry, 23.06.2019 05:30
Elizabeth has two separate samples of the same substance. sample is in the liquid state, and the other is in the solid state. the two samples most likely differ in which property?
Answers: 1
You know the right answer?
A chemist titrates 160.0 mL of a 0.3065 M cyanic acid (HCNO) solution with 0.4994 M NaOH solution at...
Questions

Mathematics, 02.11.2020 22:30



English, 02.11.2020 22:30


Mathematics, 02.11.2020 22:30



Physics, 02.11.2020 22:30

History, 02.11.2020 22:30

Mathematics, 02.11.2020 22:30

Mathematics, 02.11.2020 22:30





English, 02.11.2020 22:30


English, 02.11.2020 22:30

Mathematics, 02.11.2020 22:30



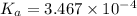


 = 98.1978 mL
= 98.1978 mL




 and
and  .
.
 of
of 




![K_{b} = \frac{[HCNO][OH^{-}]}{[CNO^{-}]}](/tpl/images/0600/3810/7fa50.png)
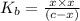




![[OH^{-}] = x = 2.34 \times 10^{-6}](/tpl/images/0600/3810/bb369.png) M
M
![-log [OH^{-}]](/tpl/images/0600/3810/337a4.png)



