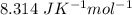Chemistry, 15.04.2020 22:40 quinteroalex2001
Calculate the value of the free energy change, ΔG, for the reaction below at 750.0ºC when the pressures of POCl3 (g) = 10.00 atm, PCl3 (g) = 0.0150 atm, and O2 (g) = 0.0100 atm.
2 POCl3 (g) →2 PCl3 (g) + O2 (g)
ΔGº = 489.75 kJ
ΔHº = 542.8 kJ
ΔSº = 177.93 J/K

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

You know the right answer?
Calculate the value of the free energy change, ΔG, for the reaction below at 750.0ºC when the pressu...
Questions








Arts, 06.12.2021 22:30

Arts, 06.12.2021 22:30

Mathematics, 06.12.2021 22:30


English, 06.12.2021 22:30





History, 06.12.2021 22:30

Chemistry, 06.12.2021 22:30

Mathematics, 06.12.2021 22:30

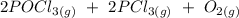
![K = \frac{[PCl_3]^2[O_2]}{[POCl_3]^2}\\\\K = \frac{(0.0150)^2*(0.0100)}{(10.00)^2}\\ \\K = 2.25*10^{-8}](/tpl/images/0603/3029/dfbf4.png)