Chemistry, 16.04.2020 02:31 hebrew1148
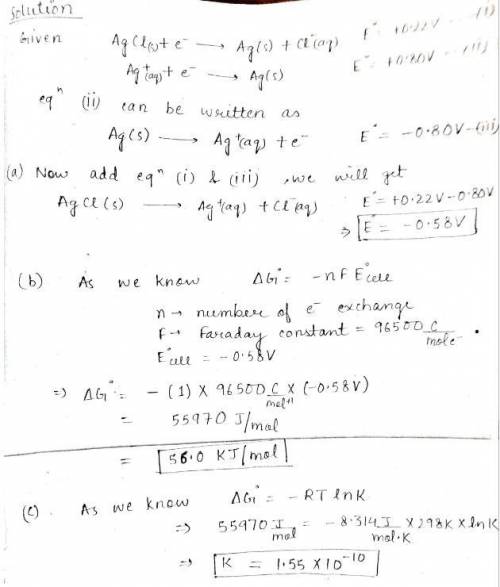
You are given the following half-reactions. AgCl(s) + e− → Ag(s) + Cl −(aq) ℰ° = +0.22 V Ag+(aq) + e− → Ag(s) ℰ° = +0.80 V Construct a cell with the following cell reaction. AgCl(s) → Ag+(aq) + Cl −(aq) Please see Adding Reactions for assistance. (a) What is the standard cell potential? WebAssign will check your answer for the correct number of significant figures. -.58 Correct: Your answer is correct. V (b) What is the value of ΔG° for the reaction? WebAssign will check your answer for the correct number of significant figures. 56 Correct: Your answer is correct. kJ/mol (c) What is the equilibrium constant as determined from the cell potential? (Please recall that often a significant figures appears to be lost when raising 10 to a power.) WebAssign will check your answer for the correct number of significant figures. 1.6e-10 Incorrect: Your answer is incorrect.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2


Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
You are given the following half-reactions. AgCl(s) + e− → Ag(s) + Cl −(aq) ℰ° = +0.22 V Ag+(aq) + e...
Questions

Biology, 11.02.2021 21:10




Arts, 11.02.2021 21:10




History, 11.02.2021 21:10


History, 11.02.2021 21:10


Computers and Technology, 11.02.2021 21:10




History, 11.02.2021 21:10