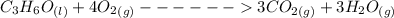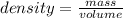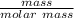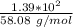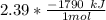Consider the thermochemical equation for the combustion of acetone (c3h6o), the main ingredient in nail polish remover. c3h6o (l) + 4 o2 (g) à 3 co2 (g) + 3 h2o (g) ∆horxn = -1790 kj if a bottle of nail polish remover contains 177 ml of acetone, how much heat is released by its complete combustion? the density of acetone is 0.788 g/ml.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Consider the thermochemical equation for the combustion of acetone (c3h6o), the main ingredient in n...
Questions

History, 11.12.2020 06:00


Mathematics, 11.12.2020 06:00

English, 11.12.2020 06:00


Mathematics, 11.12.2020 06:00


Physics, 11.12.2020 06:00

Mathematics, 11.12.2020 06:00

Mathematics, 11.12.2020 06:00

Arts, 11.12.2020 06:00

Mathematics, 11.12.2020 06:00

Chemistry, 11.12.2020 06:00

Biology, 11.12.2020 06:00



Mathematics, 11.12.2020 06:00

Mathematics, 11.12.2020 06:00


Mathematics, 11.12.2020 06:00