
Chemistry, 22.04.2020 01:27 Queenvalentin
A 25.888 g sample of aqueous waste leaving a fertilizer manufacturer contains ammonia. The sample is diluted with 73.464 g of water. A 10.762 g aliquot of this solution is then titrated with 0.1039 M HCl . It required 31.89 mL of the HCl solution to reach the methyl red endpoint. Calculate the weight percent NH3 in the aqueous waste.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Metallic bonds are good conductors of electricity true or false
Answers: 2

Chemistry, 21.06.2019 15:50
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2
You know the right answer?
A 25.888 g sample of aqueous waste leaving a fertilizer manufacturer contains ammonia. The sample is...
Questions


Mathematics, 03.02.2021 16:50

Mathematics, 03.02.2021 16:50

Biology, 03.02.2021 16:50

Chemistry, 03.02.2021 16:50


English, 03.02.2021 16:50


Social Studies, 03.02.2021 16:50





Mathematics, 03.02.2021 16:50

Mathematics, 03.02.2021 16:50

Mathematics, 03.02.2021 16:50


Mathematics, 03.02.2021 16:50

Mathematics, 03.02.2021 16:50

English, 03.02.2021 16:50


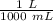
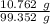
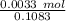
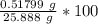 %
%

