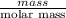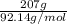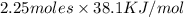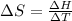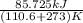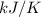Chemistry, 22.04.2020 23:45 phillipselijah2
The heat of vaporization Δ1, of toluene (C6H5CH3) is 38.1 kJ/mol. Calculate the change in entropy AS when 207. g of toluene boils at 1 10.6 °C. Be sure your answer contains a unit symbol. Round your answer to 3 significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1


Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
The heat of vaporization Δ1, of toluene (C6H5CH3) is 38.1 kJ/mol. Calculate the change in entropy AS...
Questions

English, 24.03.2021 04:20

Mathematics, 24.03.2021 04:20

Physics, 24.03.2021 04:20



Mathematics, 24.03.2021 04:20

Mathematics, 24.03.2021 04:20

Mathematics, 24.03.2021 04:20

History, 24.03.2021 04:20




Advanced Placement (AP), 24.03.2021 04:20

History, 24.03.2021 04:20


History, 24.03.2021 04:20


Social Studies, 24.03.2021 04:20

Biology, 24.03.2021 04:20

Mathematics, 24.03.2021 04:20

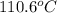 is 223
is 223  .
.