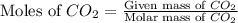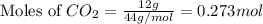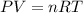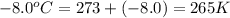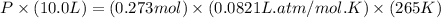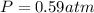Chemistry, 23.04.2020 01:05 MrTeriffic
A reaction between liquid reactants takes place at -8.0 C in a sealed, evacuated vessel with a measured volume of 10.0 L. Measurements show that the reaction produced 12 g or carbon dioxide gas. Calculate the pressure of carbon dioxide in the reaction vessel after the reaction. You may ignore the volume of the liquid reactants. Round you answer to 2 significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:50
What type of reaction is illustrated? 2c12o5 = 2cl2 + 502
Answers: 2


Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2
You know the right answer?
A reaction between liquid reactants takes place at -8.0 C in a sealed, evacuated vessel with a measu...
Questions




Mathematics, 03.04.2021 03:20


Mathematics, 03.04.2021 03:20


Mathematics, 03.04.2021 03:20


Mathematics, 03.04.2021 03:20





Mathematics, 03.04.2021 03:30


History, 03.04.2021 03:30

Mathematics, 03.04.2021 03:30

Mathematics, 03.04.2021 03:30

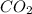 = 12 g
= 12 g