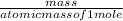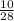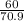Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
Si + 2Cl2 + SiC14
What mass of SiCl4 is formed when 10.0 grams of Si and 60.0
grams of C...
What mass of SiCl4 is formed when 10.0 grams of Si and 60.0
grams of C...
Questions



Social Studies, 16.10.2020 04:01

Biology, 16.10.2020 04:01

Social Studies, 16.10.2020 04:01

History, 16.10.2020 04:01



Computers and Technology, 16.10.2020 04:01



Mathematics, 16.10.2020 04:01

Chemistry, 16.10.2020 04:01



Mathematics, 16.10.2020 04:01

Physics, 16.10.2020 04:01



SAT, 16.10.2020 04:01

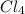 = 169.8 grams/mole
= 169.8 grams/mole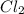 ⇒ Si
⇒ Si