Chemistry, 05.05.2020 16:32 homeworkprincess
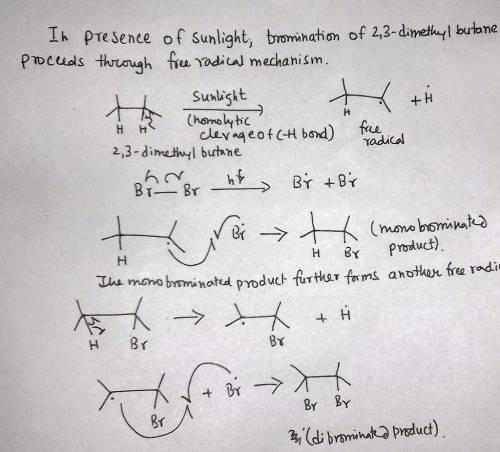
2,3-Dimethylbutane reacts with bromine in the presence of light to give a monobrominated product. Further reaction gives a good yield of a dibrominated product. Predict the structures of these products, and propose a mechanism for the formation of the monobrominated product.

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 11:30
Which part of the healthcare system could best explain how a pharmaceutical drug works
Answers: 2

Chemistry, 23.06.2019 15:30
Which term defines a type of oxygen that forms a protective layer miles above the earth a. fossil fuel b. smog c. pollution d. ozone
Answers: 2

Chemistry, 23.06.2019 16:30
Asample of calcium chloride (cacla) contains 100.2 grams of calcium (ca) and 177.3 grams of chlorine (co. what is the percent composition of chlorine 2.510% 36.10% 56.50% 63.89%
Answers: 2

Chemistry, 23.06.2019 19:30
What is the pressure of 5.0 mol nitrogen gas in a 2.0 l container at 268 k (the universal gas constant is 0.0821 l•atm/mol•k) a. 55 atm b. 8.8 atm c. 0.018 atm d. 220 atm
Answers: 1
You know the right answer?
2,3-Dimethylbutane reacts with bromine in the presence of light to give a monobrominated product. Fu...
Questions


History, 25.11.2019 21:31



Mathematics, 25.11.2019 21:31



Mathematics, 25.11.2019 21:31






Mathematics, 25.11.2019 21:31


Biology, 25.11.2019 21:31


Biology, 25.11.2019 21:31