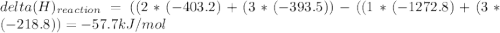Chemistry, 05.05.2020 08:30 jackiediaz
Use the given heats of formation to calculate the enthalpy change for this reaction. B2O3(g) + 3COCl2(g) →2BCl3(g) + 3CO2(g) ΔHof of B2O3(g) is –1272.8 kJ∙mol–1 ΔHof of BCl3(g) is –403.8 kJ∙mol–1 ΔHof of COCl2(g) is –218.8 kJ∙mol–1 ΔHof of CO2(g) is –393.5 kJ∙mol–1 Question 8 options: 1) 649.3 kJ·mol–1 2) 354.9 kJ·mol–1 3) –58.9 kJ·mol–1 4) –3917.3 kJ·mol–1

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1

You know the right answer?
Use the given heats of formation to calculate the enthalpy change for this reaction. B2O3(g) + 3COCl...
Questions






Mathematics, 07.04.2022 02:40




Mathematics, 07.04.2022 03:10




Mathematics, 07.04.2022 03:30


Social Studies, 07.04.2022 03:40

Mathematics, 07.04.2022 03:50