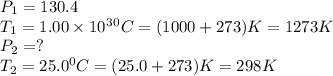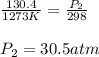Chemistry, 05.05.2020 21:33 caprisun6779
Calculate the final pressure inside a scuba tank after it cools from 1.00 x 103 °C to 25.0 °C. The initial pressure inside the tank was 130.4 atm.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1


Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
Calculate the final pressure inside a scuba tank after it cools from 1.00 x 103 °C to 25.0 °C. The i...
Questions

Chemistry, 16.10.2020 14:01


Mathematics, 16.10.2020 14:01

Mathematics, 16.10.2020 14:01








Arts, 16.10.2020 14:01


Mathematics, 16.10.2020 14:01

History, 16.10.2020 14:01

Social Studies, 16.10.2020 14:01

History, 16.10.2020 14:01


Mathematics, 16.10.2020 14:01

Computers and Technology, 16.10.2020 14:01

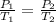
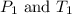 are the initial pressure and temperature of the gas.
are the initial pressure and temperature of the gas.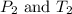 are the final pressure and temperature of the gas.
are the final pressure and temperature of the gas.