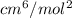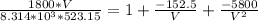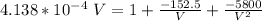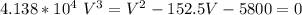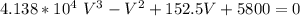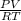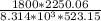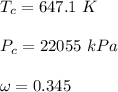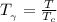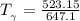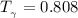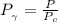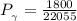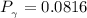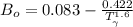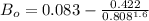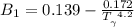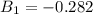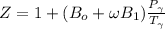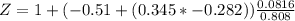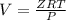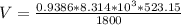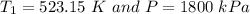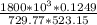Chemistry, 06.05.2020 04:46 andresduenas72
Determine Z and V for steam at 250°C and 1800 kPa by the following: (a) The truncated virial equation [Eq. (3.38)] with the following experimental values of virial coefficients: B = −152.5 cm3·mol−1 C = −5800 cm6·mol−2 (b) The truncated virial equation [Eq. (3.36)], with a value of B from the generalized Pitzer correlation [Eqs. (3.58)–(3.62)]. (c) The steam tables (App. E).

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2

Chemistry, 23.06.2019 06:40
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1
You know the right answer?
Determine Z and V for steam at 250°C and 1800 kPa by the following: (a) The truncated virial equatio...
Questions


Mathematics, 25.02.2020 22:26















Health, 25.02.2020 22:26



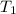 = 250 °C= ( 250+ 273.15 ) K = 523.15 K
= 250 °C= ( 250+ 273.15 ) K = 523.15 K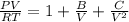
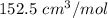 C = -5800
C = -5800