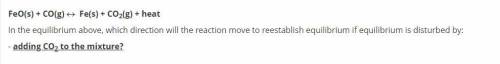
FeO(s) + CO(g) « Fe(s) + CO2(g) + heat
In the equilibrium above, which direction will the reac...


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3
You know the right answer?
Questions


Mathematics, 28.05.2021 17:00






Computers and Technology, 28.05.2021 17:00




Computers and Technology, 28.05.2021 17:00








Mathematics, 28.05.2021 17:00