
Chemistry, 19.05.2020 03:17 Redhead667
1.What mass of oxygen gas, O2, from the air is consumed in the combustion of 702g of octane, C8H18, one of the principal components of gasoline? 2C8H18 + 25O2 -> 16CO2 + 18H2O.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
1.What mass of oxygen gas, O2, from the air is consumed in the combustion of 702g of octane, C8H18,...
Questions

Biology, 21.01.2020 23:31

Biology, 21.01.2020 23:31

Biology, 21.01.2020 23:31


Computers and Technology, 21.01.2020 23:31















History, 21.01.2020 23:31

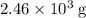 .
.  :
: 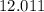 .
. :
: 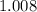 .
. :
: 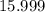 .
.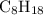 :
: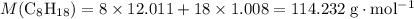 .
.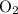 :
: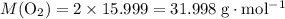 .
.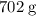 of octane,
of octane, 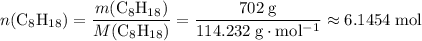 .
.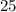 , whileThe coefficient of
, whileThe coefficient of 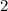 .
.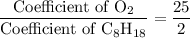 .
.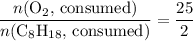 .
.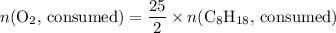 .
.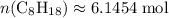 . Hence, the number of moles of oxygen gas required will be:
. Hence, the number of moles of oxygen gas required will be: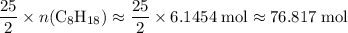 .
.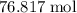 of
of 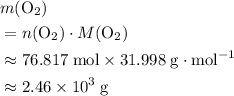 .
.

